Ανάλυση: Τα αντιυπερτασικά φάρμακα δεν καθιστούν τους ασθενείς πιο ευάλωτους στη λοίμωξη από κορωνοϊό
04/04/2020
Η πανδημία του κορωνοϊού έχει προκαλέσει πολλά ερωτήματα και έχει πυροδοτήσει την έρευνα για τους βιολογικούς μηχανισμούς διείσδυσης του ιού μέσα στα κύτταρα του ανθρώπινου οργανισμού.
Πρόσφατα δημοσιεύτηκαν στο έγκυρο περιοδικό Nature τα αποτελέσματα μια πειραματικής μελέτης του καθηγητή Φανγκ Λι, σύμφωνα με την οποία ο κορωνοϊός χρησιμοποιεί τους υποδοχείς του μετατρεπτικού ενζύμου της αγγειοτανσίνης (ACE 2) που εδράζονται στα κυψελιδικά κύτταρα των πνευμόνων. Ουσιαστικά οι ερευνητές διατείνονται ότι οι υποδοχείς ACE 2 αποτελούν τον «Δούρειο Ίππο» δια μέσω του οποίου ο κορωνοϊός διεισδύει μέσα στα ανθρώπινα κύτταρα και εν συνεχεία πολλαπλασιάζεται με ταχείς ρυθμούς. Στους ασθενείς που νοσούν αν και προεξέχουν τα συμπτώματα από το αναπνευστικό σύστημα, ιδίως πνευμονία και κατ΄επέκταση βαρεία αναπνευστική ανεπάρκεια, εντούτοις συχνά καταγράφονται μυοκαρδίτιδες, αρρυθμίες και νεφρική ανεπάρκεια, ευρήματα που σχετίζονται με την εκτεταμένη δράση του ACE 2.
Όμως τι είναι το ΑCE και ποιος είναι η βιολογική του δράση;
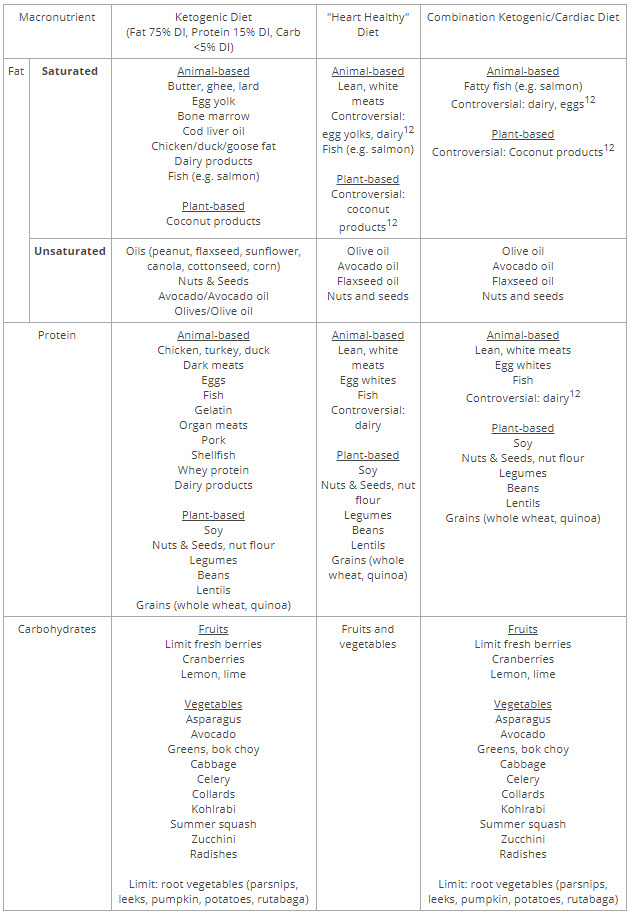
Στον ανθρώπινο οργανισμό υπάρχουν πολλαπλοί νευρο-ορμονικοί μηχανισμοί ρύθμισης της αρτηριακής πίεσης και της διατήρησης ισορροπίας υγρών και ηλεκτρολυτών. Ο βασικότερος μηχανισμός είναι το σύστημα ρενίνης-αγγειοτενσίνης-αλδοστερόνης.
Η ρενίνη παράγεται στους νεφρούς και μετατρέπει το αγγειοτενσινογόνο σε αγγειοτενσίνη Ι (δεκαπεπτίδιο). Εν συνεχεία το μετατρεπτικό ένζυμο της αγγειοτενσίνης (ACE) μετατρέπει την αγγειοτενσίνη Ι σε αγγειοτενσίνη ΙΙ (οκταπεπτίδιο).
Η αγγειοτενσίνη ΙΙ δρα ως ορμόνη που προκαλεί:
α) σύσπαση των αγγείων με αποτέλεσμα αύξηση της αρτηριακής πίεσης.
β) απελευθέρωση της αλδοστερόνης από τα επινεφρίδια. Η αλδοστερόνη προκαλεί κατακράτηση νατρίου και υγρών και κατά συνέπεια αυξάνει την αρτηριακή πίεση.
Tη δεκαετία του ’80 αναπτύχθηκαν οι αναστολείς του μετατρεπτικού ενζύμου της αγγειοτανσίνης (α-ΜΕΑ), ισχυρά και αποτελεσματικά φάρμακα που αποτέλεσαν επανάσταση στη θεραπεία της υπέρτασης. Μέχρι τότε η αγωγή βασιζόταν στη χορήγηση μεγάλων δόσεων διουρητικών και σε κεντρικώς δρώντα φάρμακα (δρούσαν σε υποδοχείς του εγκεφάλου), που είχαν πολλαπλές παρενέργειες και δεν είχαν εφάμιλλη αντιϋπερτασική δράση.
Τη δεκαετία του ΄90 αναπτύχθηκαν οι αναστολείς των υποδοχέων της αγγειοτανσίνης ΙΙ (σαρτάνες), επίσης αποτελεσματικά και ασφαλή φάρμακα. Οι δύο παραπάνω κατηγορίες φαρμάκων δοκιμάστηκαν με μεγάλη επιτυχία και στη θεραπεία ασθενών με καρδιακή ανεπάρκεια, ιδίως όσων είχαν υποστεί εκτεταμένο έμφραγμα μυοκαρδίου.
Οι πρώτες επιδημιολογικές μελέτες από την πόλη Wuhan της Κίνας, από όπου ξεκίνησε η πανδημία, κατέδειξαν ότι το 60% περίπου των ασθενών που νοσηλευόντουσαν στις μονάδες εντατικής θεραπείας και το 60% των ασθενών που κατέληξαν λαμβάναν αντιϋπερτασικά φάρμακα, γεγονός που έχει πυροδοτήσει τον επιστημονικό διάλογο για την ασφάλεια των προαναφερθέντων φαρμάκων (α-ΜΕΑ και σαρτάνες).
Θα πρέπει να γνωρίζουμε ότι οι υποδοχείς ACE 2 έχουν συγγένεια μόνο κατά 42% με τους αντίστοιχους του ΑCE. Επιπρόσθετα το υπόστρωμα και η δραστικότητα των δύο ενζύμων διαφοροποιείται σημαντικά. Τέλος δεν είναι τεκμηριωμένο ότι τα φάρμακα αποκλείουν τους υποδοχείς ACE 2 που εδράζονται στους πνεύμονες.
Δεν θα πρέπει να λησμονούμε το γεγονός ότι οι ασθενείς που λαμβάναν αντιϋπερτασικά συνηθέστερα έχουν συνοσηρρότητες (π.χ. διαβήτης) ή πιθανόν έχουν υποστεί στο παρελθόν έμφραγμα μυοκαρδίου-αγγειακό εγκεφαλικό επεισόδιο.
Πάντως ακόμη δε γνωρίζουμε αν η υπέρταση είναι πραγματικός παράγοντας κινδύνου (ανεξάρτητα από την ηλικία) για την ανάπτυξη της λοίμωξης από κορωνοϊό ή ένας παράγοντας που καθορίζει τη σοβαρότητα της ασθένειας. Δεν υπάρχουν κλινικά δεδομένα για να υποστηριχθεί ότι η λήψη α-ΜΕΑ/σαρτατών αυξάνει τον κίνδυνο και ούτε επιστημονικά δεδομένα τεκμηριώνουν μία σαφή συσχέτιση.
Το μόνο βέβαιο είναι ότι το ACE 2 αποτελεί σαφή θεραπευτικό στόχο και ήδη διεξάγεται κλινική μελέτη στην Κίνα με χορήγηση ανασυνδυασμένου ΑCE 2 από ανθρώπινα κύτταρα.
Με βάση τα παραπάνω, στην τρέχουσα φάση τόσο η Ελληνική Καρδιολογική Εταιρεία (ΕΚΕ) όσο και η Ευρωπαϊκή Καρδιολογική Εταιρεία (ESC) αλλά και η Αμερικάνικη Καρδιολογική Εταιρεία (ΑΗΑ) δεν συνιστούν διακοπή ή αλλαγή των αντιϋπερτασικών φαρμάκων, τονίζοντας ότι είναι αναγκαία περαιτέρω έρευνα για την επίδραση της υπέρτασης και της αντιϋπερτασικής θεραπείας στην έκβαση των ασθενών που μολύνθηκαν από κορωνοϊό.
Ειδικότερα η διακοπή της θεραπείας σε ασθενείς με καρδιακή ανεπάρκεια ή ιστορικό εμφράγματος μυοκαρδίου εγκυμονεί κίνδυνο απορρύθμισης και δυσμενούς έκβασης.
Επισημαίνεται ότι ομάδες υψηλού κινδύνου σε περίπτωση λοίμωξης από κορωνοϊό θεωρούνται:
α) ηλικιωμένοι ασθενείς
β) ανοσοκατασταλμένοι ασθενείς (π.χ. καρκινοπαθείς, υπό κορτιζόνη, αυτοάνοσα νοσήματα).
γ) καρδιαγγειακά νοσήματα (ιδίως καρδιακή ανεπάρκεια, μη ρυθμιζόμενος σακχαρώδης διαβήτης σε συνδυασμό με άλλα νοσήματα)
δ) ασθενείς με χρόνια αποφρακτική πνευμονοπάθεια.
Συνοψίζοντας, τα τρέχοντα επιστημονικά δεδομένα συγκλείνουν στη συνέχιση της φαρμακευτικής αγωγής με αντιϋπερτασικά φάρμακα (α-ΜΕΑ, σαρτάνες) τόσο στους ασθενείς που νοσούν από κορωνοϊό όσο και στους ασθενείς υψηλού κινδύνου.
* Χρήστος Καϊρης, Καρδιολόγος, Δράμα -chriskairis@hotmail.com
* Γιάννης Γουδέβενος, Καθηγητής Καρδιολογίας, Πρόεδρος της Ελληνικής Καρδιολογικής Εταιρείας – igoudev@gmail.com
Πηγή: kathimerini.gr